상품 정보
상품 기본설명
상품 상세설명
PureDireX Genomic DNA Isolation Dual Kit (Column Based)
The DUAL Genomic DNA Isolation Kit (Blood/ Cultured Cell/ Fungus) combines the reagent system and spin column system. The kit is designed specifically for isolating the genomic DNA from the whole blood, frozen blood, buffy coat, cultured animal/ bacterial cells, and fungus. This unique reagent system ensures the total DNA with a high yield and good quality from the samples. The spin column system is designed to purify or concentrate DNA products which have been previously isolated with the reagents. The entire procedure can be completed in 1 hour without the phenol/ chloroform extraction. The purified DNA is suitable for use in PCR or other enzymatic reactions.
Sample
Up to 300 μl of the whole blood
Up to 200 μl of the frozen blood
Up to 200 μl of the buffy coat
Cultured animal cells (up to 1 x10^7)
Cultured bacterial cells (up to 1 x10^9)
Fungus cells (up to 5 x 10^7)
Format
Reagent and spin column
Yield
Up to 50 μg
Operation time
Within 60 minutes
Elution volume
50∼200 μl
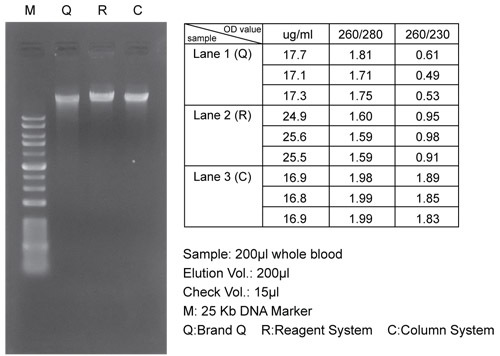
[PureDireX / PDC02-0100 / Bio-Helix]
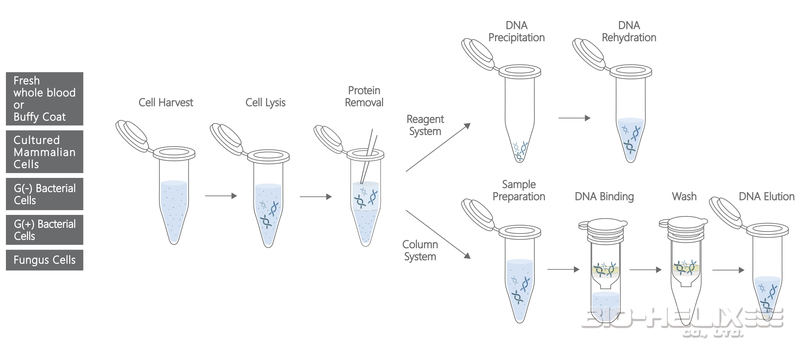
▍Fresh Whole Blood or Buffy Coat
Reagent System Protocol
Step 1 - Sample Cells Harvesting
1. Collect blood in the EDTA-Na2 treated collection tubes (or other anticoagulant mixtures).
2. Transfer up to 300 µl of the blood or 200 µl of buffy coat to a sterile 1.5 ml microcentrifuge tube.
3. Add 900 µl of the Buffer RL and mix by inversion.
4. Incubate the tube at the room temperature for 10 minutes (invert twice during incubation).
5. Centrifuge at 4,000 x g for 5 minutes.
6. Remove the supernatant completely and resuspend the cells in 50 µl of the Buffer RL by pipetting the pellet.
Step 2 - Lysis
1. Add 300 µl of the Buffer CL to the resuspended cells from Step 1 and mix by vortex.
2. Incubate at 60°C for 10 minutes or until the sample lysate is clear. During the incubation, invert the tube every 3 minutes.
Optional Step: RNA Degradation (If RNA-free genomic DNA is required, perform this optional step.)
3. Add 5 µl of RNase A (10 mg/ml) to the sample lysate and mix by vortex. Incubate at room temperature for 5 minutes.
Step 3 - Protein Removal
1. Add 100 µl of the Buffer PO to the sample lysate and vortex immediately for 10 seconds.
2. Incubate on ice for 5 minutes.
3. Centrifuge at 14-16,000 x g for 3 minutes.
4. Transfer the supernatant to a clean 1.5 ml microcentrifuge tube.
Switch Step
◆ If more pure DNA is required, please switch to Column System (DNA Pure) Protocol.
Step 4 - DNA Precipitation
1. Add 300 µl of Isopropanol to the sample from the Step 3 and mix well by inverting 20 times.
2. Centrifuge at 14-16,000 x g for 5 minutes.
3. Discard the supernatant and add 300 µl of 70% ethanol to wash the pellet.
4. Centrifuge at 14-16,000 x g for 3 minutes.
5. Discard the supernatant and air-dry the pellet for 10 minutes.
Step 5 DNA Rehydration
1. Add 50-100 µl of the Buffer E and incubate at 60°C for 5-10 minutes to dissolve the DNA pellet. During the incubation, tap the bottom of the tube to promote DNA rehydration.
Column System (DNA Pure) Protocol
* Add 60ml of the absolute ethanol to the Buffer W2 prior to initial use.
* Pre-heat the Buffer E to 60°C prior to use.
Step 1 - Sample Preparation
1. Add 400 µl of the Buffer BD to the sample from Step 3 Protein Removal and shake vigorously.
Step 2 - DNA Binding
1. Place a DG Column in a 2 ml Collection Tube.
2. Transfer the sample mixture from the previous step to the DG Column.
3. Centrifuge at 14-16,000 x g for 30 seconds.
4. Discard the flow-through and place the DG Column back in the same Collection Tube.
Step 3 - Wash
1. Add 400 µl of the Buffer W1 into the DG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the DG Column back into the same Collection tube.
4. Add 600 µl of the Buffer W2 (Ethanol added) into the DG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the DG Column back into the same Collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove residual Buffer W2.
Step 4 - DNA Elution
1. Place the dried DG column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 µl of Pre-Heated Buffer E or TE (not provided) into the center of the column matrix.
3. Let it stand at 60°C for 5 minutes.
4. Centrifuge for 2 minutes at 14-16,000 x g to elute the purified DNA.
▍Cultured Mammalian Cells
Reagent System Protocol
Step 1 - Sample Cells Harvesting
1. Transfer cultured mammalian cells (up to 10^7) to a sterile 1.5 ml microcentrifuge tube.
2. Centrifuge at 6,000 x g for 1 minute.
3. Remove the supernatant completely and resuspend the cells in 50 µl of the Buffer RL by pipetting the pellet.
Step 2 - Lysis
1. Add 300 µl of the Buffer CL to the resuspended cells from Step 1 and mix by vortex.
2. Incubate at 60°C for 10 minutes or until the sample lysate is clear. During the incubation, invert the tube every 3 minutes.
Optional Step: RNA Degradation (If RNA-free genomic DNA is required, perform this optional step.)
3. Add 5 µl of RNase A (10 mg/ml) to the sample lysate and mix by vortex. Incubate at room temperature for 5 minutes.
Step 3 - Protein Removal
1. Add 100 µl of the Buffer PO to the sample lysate and vortex immediately for 10 seconds.
2. Incubate on ice for 5 minutes.
3. Centrifuge at 14-16,000 x g for 3 minutes.
4. Transfer the supernatant to a clean 1.5 ml microcentrifuge tube.
Switch Step
◆If more pure DNA is required, please switch to Column System (DNA Pure) Protocol.
Step 4 - DNA Precipitation
1. Add 300 µl of Isopropanol to the sample from the Step 3 and mix well by inverting 20 times.
2. Centrifuge at 14-16,000 x g for 5 minutes.
3. Discard the supernatant and add 300 µl of 70% ethanol to wash the pellet.
4. Centrifuge at 14-16,000 x g for 3 minutes.
5. Discard the supernatant and air-dry the pellet for 10 minutes.
Step 5 - DNA Rehydration
1. Add 50-100 µl of the Buffer E and incubate at 60°C for 5-10 minutes to dissolve the DNA
pellet. During the incubation, tap the bottom of the tube to promote DNA rehydration.
Column System (DNA Pure) Protocol
* Add 60ml of the absolute ethanol to the Buffer W2 prior to initial use.
* Pre-heat the Buffer E to 60°C prior to use.
Step 1 - Sample Preparation
1. Add 400 µl of the Buffer BD to the sample from Step 3 Protein Removal and shake vigorously.
Step 2 - DNA Binding
1. Place a DG Column in a 2 ml Collection Tube.
2. Transfer the sample mixture from the previous step to the DG Column.
3. Centrifuge at 14-16,000 x g for 30 seconds.
4. Discard the flow-through and place the DG Column back in the same Collection Tube.
Step 3 - Wash
1. Add 400 µl of the Buffer W1 into the DG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the DG Column back into the same Collection tube.
4. Add 600 µl of the Buffer W2 (Ethanol added) into the DG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the DG Column back into the same Collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove residual Buffer W2.
Step 4 - DNA Elution
1. Place the dried DG column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 µl of Pre-Heated Buffer E or TE (not provided) into the center of the column matrix.
3. Let it stand at 60°C for 5 minutes.
4. Centrifuge for 2 minutes at 14-16,000 x g to elute the purified DNA.
▍Gram-Negative Bacterial Cells
Reagent System Protocol
Step 1 - Sample Cells Harvesting
1. Transfer cultured bacterial cells (up to 10^9) to a sterile 1.5 ml microcentrifuge tube.
2. Centrifuge at 12,000 x g for 1 minute.
3. Remove the supernatant completely and resuspend the cells in 50 µl of the Buffer RL by pipetting the pellet.
Step 2 - Lysis
1. Add 300 µl of the Buffer CL to the resuspended cells from Step 1 and mix by vortex.
2. Incubate at 60°C for 10 minutes or until the sample lysate is clear. During the incubation, invert the tube every 3 minutes.
Optional Step: RNA Degradation (If RNA-free genomic DNA is required, perform this optional step.)
3. Add 5 µl of RNase A (10 mg/ml) to the sample lysate and mix by vortex. Incubate at room temperature for 5 minutes.
Step 3 - Protein Removal
1. Add 100 µl of the Buffer PO to the sample lysate and vortex immediately for 10 seconds.
2. Incubate on ice for 5 minutes.
3. Centrifuge at 14-16,000 x g for 3 minutes.
4. Transfer the supernatant to a clean 1.5 ml microcentrifuge tube.
Switch Step
◆If more pure DNA is required, please switch to Column System (DNA Pure) Protocol.
Step 4 - DNA Precipitation
1. Add 300 µl of Isopropanol to the sample from the Step 3 and mix well by inverting 20 times.
2. Centrifuge at 14-16,000 x g for 5 minutes.
3. Discard the supernatant and add 300 µl of 70% ethanol to wash the pellet.
4. Centrifuge at 14-16,000 x g for 3 minutes.
5. Discard the supernatant and air-dry the pellet for 10 minutes.
Step 5 - DNA Rehydration
1. Add 50-100 µl of the Buffer E and incubate at 60°C for 5-10 minutes to dissolve the DNA pellet. During the incubation, tap the bottom of the tube to promote DNA rehydration.
Column System (DNA Pure) Protocol
* Add 60ml of the absolute ethanol to the Buffer W2 prior to initial use.
* Pre-heat the Buffer E to 60°C prior to use.
Step 1 - Sample Preparation
1. Add 400 µl of the Buffer BD to the sample from Step 3 Protein Removal and shake vigorously.
Step 2 - DNA Binding
1. Place a DG Column in a 2 ml Collection Tube.
2. Transfer the sample mixture from the previous step to the DG Column.
3. Centrifuge at 14-16,000 x g for 30 seconds.
4. Discard the flow-through and place the DG Column back in the 2 ml Collection Tube.
Step 3 - Wash
1. Add 400 µl of the Buffer W1 into the DG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the DG Column back into the same Collection tube.
4. Add 600 µl of the Buffer W2 (Ethanol added) into the DG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the DG Column back into the same Collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove residual Buffer W2.
Step 4 - DNA Elution
1. Place the dried DG column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 µl of Pre-Heated Buffer E or TE (not provided) into the center of the column matrix.
3. Let it stand at 60°C for 5 minutes.
4. Centrifuge for 2 minutes at 14-16,000 x g to elute the purified DNA.
▍Gram-Postive Bacterial Cells
Reagent System Protocol
Step 1 - Sample Cells Harvesting
1. Transfer cultured bacterial cells (up to 10^9) to a sterile 1.5 ml microcentrifuge tube.
2. Centrifuge at 12,000 x g for 1 minute.
3. Remove the supernatant completely and resuspend the cells in 100 µl of lysozyme Buffer by pipetting the pellet.
4. Incubate at room temperature for 20 minutes.
Step 2 - Lysis
1. Add 300 µl of the Buffer CL to the resuspended cells from Step 1 and mix by vortex.
2. Incubate at 60°C for 10 minutes or until the sample lysate is clear. During the incubation, invert the tube every 3 minutes.
Optional Step: RNA Degradation (If RNA-free genomic DNA is required, perform this optional step.)
3. Add 5 µl of RNase A (10 mg/ml) to the sample lysate and mix by vortex. Incubate at room temperature for 5 minutes.
Step 3 - Protein Removal
1. Add 100 µl of the Buffer PO to the sample lysate and vortex immediately for 10 seconds.
2. Incubate on ice for 5 minutes.
3. Centrifuge at 14-16,000 x g for 3 minutes.
4. Transfer the supernatant to a clean 1.5 ml microcentrifuge tube.
Switch Step
◆If more pure DNA is required, please switch to Column System (DNA Pure) Protocol.
Step 4 - DNA Precipitation
1. Add 300 µl of Isopropanol to the sample from the Step 3 and mix well by inverting 20 times.
2. Centrifuge at 14-16,000 x g for 5 minutes.
3. Discard the supernatant and add 300 µl of 70% ethanol to wash the pellet.
4. Centrifuge at 14-16,000 x g for 3 minutes.
5. Discard the supernatant and air-dry the pellet for 10 minutes.
Step 5 - DNA Rehydration
1. Add 50-100 µl of the Buffer E and incubate at 60°C for 5-10 minutes to dissolve the DNA pellet. During the incubation, tap the bottom of the tube to promote DNA rehydration.
Column System (DNA Pure) Protocol
* Add 60ml of the absolute ethanol to the Buffer W2 prior to initial use.
* Pre-heat the Buffer E to 60°C prior to use.
Step 1 - Sample Preparation
1. Add 400 µl of the Buffer BD to the sample from Step 3 Protein Removal and shake vigorously.
Step 2 - DNA Binding
1. Place a DG Column in a 2 ml Collection Tube.
2. Transfer the sample mixture from the previous step to the DG Column.
3. Centrifuge at 14-16,000 x g for 30 seconds.
4. Discard the flow-through and place the DG Column back in the 2 ml Collection Tube.
Step 3 - Wash
1. Add 400 µl of the Buffer W1 into the DG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the DG Column back into the same Collection tube.
4. Add 600 µl of the Buffer W2 (Ethanol added) into the DG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the DG Column back into the same Collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove residual Buffer W2.
Step 4 - DNA Elution
1. Place the dried DG column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 µl of Pre-Heated Buffer E or TE (not provided) into the center of the column matrix.
3. Let it stand at 60°C for 5 minutes.
4. Centrifuge for 2 minutes at 14-16,000 x g to elute the purified DNA.
▍Fungus Cells
Reagent System Protocol
Step 1 - Sample Cells Harvesting
1. Transfer fungus cells (up to 10^8) to a sterile 1.5 ml microcentrifuge tube.
2. Centrifuge at 6,000 x g for 5 minutes.
3. Remove the supernatant completely and resuspend the cells in 600 µl of sorbitol Buffer by pipetting the pellet.
4. Add 200 U of lyticase or zymolase. Incubate at 30°C for 30 minutes.
5. Centrifuge the mixture for 10 minutes at 2,000 x g to harvest the spheroplast.
6. Remove the supernatant completely and resuspend the cells in 50 µl of the Buffer RL by pipetting the pellet.
Step 2 - Lysis
1. Add 300 µl of the Buffer CL to the resuspended cells from Step 1 and mix by vortex.
2. Incubate at 60°C for 10 minutes or until the sample lysate is clear. During the incubation, invert the tube every 3 minutes.
Optional Step: RNA Degradation (If RNA-free genomic DNA is required, perform this optional step.)
3. Add 5 µl of RNase A (10 mg/ml) to the sample lysate and mix by vortex. Incubate at room temperature for 5 minutes.
Step 3 - Protein Removal
1. Add 100 µl of the Buffer PO to the sample lysate and vortex immediately for 10 seconds.
2. Incubate on ice for 5 minutes.
3. Centrifuge at 14-16,000 x g for 3 minutes.
4. Transfer the supernatant to a clean 1.5 ml microcentrifuge tube.
Switch Step
◆If more pure DNA is required, please switch to Column System (DNA Pure) Protocol.
Step 4 - DNA Precipitation
1. Add 300 µl of Isopropanol to the sample from the Step 3 and mix well by inverting 20 times.
2. Centrifuge at 14-16,000 x g for 5 minutes.
3. Discard the supernatant and add 300 µl of 70% ethanol to wash the pellet.
4. Centrifuge at 14-16,000 x g for 3 minutes.
5. Discard the supernatant and air-dry the pellet for 10 minutes.
Step 5 - DNA Rehydration
1. Add 50-100 µl of the Buffer E and incubate at 60°C for 5-10 minutes to dissolve the DNA pellet. During the incubation, tap the bottom of the tube to promote DNA rehydration.
Column System (DNA Pure) Protocol
* Add 60ml of the absolute ethanol to the Buffer W2 prior to initial use.
* Pre-heat the Buffer E to 60°C prior to use.
Step 1 - Sample Preparation
1. Add 400 µl of the Buffer BD to the sample from Step 3 Protein Removal and shake vigorously.
Step 2 - DNA Binding
1. Place a DG Column in a 2 ml Collection Tube.
2. Transfer the sample mixture from the previous step to the DG Column.
3. Centrifuge at 14-16,000 x g for 30 seconds.
4. Discard the flow-through and place the DG Column back in the 2 ml Collection Tube.
Step 3 - Wash
1. Add 400 µl of the Buffer W1 into the DG Column.
2. Centrifuge at 14,000 x g for 30 seconds.
3. Discard the flow-through and place the DG Column back into the same Collection tube.
4. Add 600 µl of the Buffer W2 (Ethanol added) into the DG Column.
5. Centrifuge at 14,000 x g for 30 seconds.
6. Discard the flow-through and place the DG Column back into the same Collection tube.
7. Centrifuge at 14,000 x g again for 2 minutes to remove residual Buffer W2.
Step 4 - DNA Elution
1. Place the dried DG column in a clean 1.5 ml microcentrifuge tube.
2. Add 50-200 µl of Pre-Heated Buffer E or TE (not provided) into the center of the column matrix.
3. Let it stand at 60°C for 5 minutes.
4. Centrifuge for 2 minutes at 14-16,000 x g to elute the purified DNA.
상품문의
등록된 상품문의
상품문의가 없습니다.
반품/교환정보
셀젠바이오샵에서는 다음과 같은 기간 및 내용으로 상품에 대하여 교환, 반품, 환불을 보장하고 있으며, 상품의 반환에 의한추가비용을 고객에게 부담시지키 않습니다. (단, 고객 변심 또는 주문 반복으로 인한 경우의 반환비용은 고객님이 부담하셔야
합니다.)
::: 교환 및 반품이 가능한 경우:::
단, 상품을 개봉하여 상품가치가 상실된 경우에는 교환/반품이 불가능합니다.
:::교환 및 반품이 불가능한 경우:::
주문 취소 및 반품으로 환불을 요청하실 경우에는 E-mail(celgen-bio@celgen-bio.com)이나 고객만족센터 (042-824-9026)을
통해 요청하시면 친절하게 처리해 드리겠습니다.
주문 취소 후 반품 가능 여부를 확인한 다음 3일 이내에 결제 금액을 환불해 드리겠습니다.